- صفحه اصلی
- محصولات
- محصولات
- Cathodic Protection System
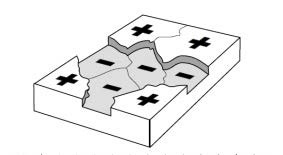
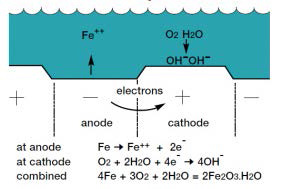
- How Does Steel Corrode?
- How Does Cathodic Protection Stop Corrosion?
- Cathodic Protection Systems
- Cathodic Protection Ground beds
- ICCP Equipment
- Anodes
- Backfill
- Anode Centralizer
- Galvanic System Equipment
- Other Equipment
- Zinc Earthing Cell
- Polarization Cell
- Insulating Flange Kits
- Cable Connecting
- Cathodic Protection Engineering Services
- Code and Standard
- Exothermic Welding System
- The Exothermic Weld Process
- Making a Exothermic weld joint is a simple procedure as illustrated below
- Accessories For Exothermic Weld
- Types of connections
- Rutin Connections Details
- Cable to Steel PIPE Connection
- Cable to Steel PIPE Connection Run Type
- Tape To Tape Connection – Straight Type – Vertical
- Tape To Tape Connection – Straight Type – Horizontal
- Tape to Tape Connection –“T” Type Horizontal
- Cable to Tape connection – Straight Type
- Cable to Tape Connection –“T” Type
- Cable to Cable Connection Straight Type
- Cable to Cable Connection – “T” Type
- Cable to Cable Connection -Interapted Cross Type
- Cable to Cable Connection – Parallel Type – Vertical
- Cable to Cable Connection – Pussing cross Type
- Cable to Rod Connection –“T” Connection
- Cable to Rod Connection – Cross Type
- Cable to Rod Connection – “L” Type
- Cable to Reinforcing Rebar Connection –“T” Type
- Cable to Reinforcing Rebar Connection –“L” Type
- Cable to Reinforcing Rebar Connection –Parallel Type
- Cable to Steel Surface Connection
- Cable to Steel Surface Horizontal Connection – Run Type
- Cable to Steel Surface Vertical Connection – Run Type
- Cable to Steel Surface Vertical Connection – Upwards Type
- Tape to Steel Surface Connection – Upwards Tape
- Inspection of ZINOWELD Connections Visual Inspection
- Lightning Protection System
- Damage Caused by Surge Voltages
- Operation of the starting apparatus to lightning ESE
- Lightning Rod ESE 1
- Lightning Rod ESE 2
- Comparison of the effectiveness of protective solutions against lightning
- Contact + Dongle
- Contact + Rout
- Reception of information
- Mast for Lightning
- Lightning Air Rod
- Saddles For Air Rod
- Saddles for side wall
- Down Conductors Holders
- Tape Clamp
- Test Clamps
- Lightning protection for floating roof tanks
- Surge arrester
- What are transient surges?
- Causes of lightning currents
- Gradual surge reduction with lightning protection zones
- Zone transitions and protective devices
- Zone transitions
- Installation instructions
- Cable networks, TN-C network system
- Cable networks, TN-S and TT network system
- Installation location
- Overview
- Earthing System
- Copper bond Earth Rods
- Solid Copper Earth Rods
- Stainless Steel Earth Rods
- Earthing clamp connection to rod
- Connection to Structure
- Connectors to connectors clamp
- High Concrete Earthing Connection
- Connection to Steel Structures
- Compression Terminal
- Accessories For Earthing Well
- Earthing Busbar
- Copper Tapes
- Cable
- Solar System
- Cathodic Protection System
- بررسی کلی محصولات
- محصولات
- کتابخانه
- درباره ما
- تماس با ما
- صفحه اصلی
- محصولات
- محصولات
- Cathodic Protection System
- How Does Steel Corrode?
- How Does Cathodic Protection Stop Corrosion?
- Cathodic Protection Systems
- Cathodic Protection Ground beds
- ICCP Equipment
- Anodes
- Backfill
- Anode Centralizer
- Galvanic System Equipment
- Other Equipment
- Zinc Earthing Cell
- Polarization Cell
- Insulating Flange Kits
- Cable Connecting
- Cathodic Protection Engineering Services
- Code and Standard
- Exothermic Welding System
- The Exothermic Weld Process
- Making a Exothermic weld joint is a simple procedure as illustrated below
- Accessories For Exothermic Weld
- Types of connections
- Rutin Connections Details
- Cable to Steel PIPE Connection
- Cable to Steel PIPE Connection Run Type
- Tape To Tape Connection – Straight Type – Vertical
- Tape To Tape Connection – Straight Type – Horizontal
- Tape to Tape Connection –“T” Type Horizontal
- Cable to Tape connection – Straight Type
- Cable to Tape Connection –“T” Type
- Cable to Cable Connection Straight Type
- Cable to Cable Connection – “T” Type
- Cable to Cable Connection -Interapted Cross Type
- Cable to Cable Connection – Parallel Type – Vertical
- Cable to Cable Connection – Pussing cross Type
- Cable to Rod Connection –“T” Connection
- Cable to Rod Connection – Cross Type
- Cable to Rod Connection – “L” Type
- Cable to Reinforcing Rebar Connection –“T” Type
- Cable to Reinforcing Rebar Connection –“L” Type
- Cable to Reinforcing Rebar Connection –Parallel Type
- Cable to Steel Surface Connection
- Cable to Steel Surface Horizontal Connection – Run Type
- Cable to Steel Surface Vertical Connection – Run Type
- Cable to Steel Surface Vertical Connection – Upwards Type
- Tape to Steel Surface Connection – Upwards Tape
- Inspection of ZINOWELD Connections Visual Inspection
- Lightning Protection System
- Damage Caused by Surge Voltages
- Operation of the starting apparatus to lightning ESE
- Lightning Rod ESE 1
- Lightning Rod ESE 2
- Comparison of the effectiveness of protective solutions against lightning
- Contact + Dongle
- Contact + Rout
- Reception of information
- Mast for Lightning
- Lightning Air Rod
- Saddles For Air Rod
- Saddles for side wall
- Down Conductors Holders
- Tape Clamp
- Test Clamps
- Lightning protection for floating roof tanks
- Surge arrester
- What are transient surges?
- Causes of lightning currents
- Gradual surge reduction with lightning protection zones
- Zone transitions and protective devices
- Zone transitions
- Installation instructions
- Cable networks, TN-C network system
- Cable networks, TN-S and TT network system
- Installation location
- Overview
- Earthing System
- Copper bond Earth Rods
- Solid Copper Earth Rods
- Stainless Steel Earth Rods
- Earthing clamp connection to rod
- Connection to Structure
- Connectors to connectors clamp
- High Concrete Earthing Connection
- Connection to Steel Structures
- Compression Terminal
- Accessories For Earthing Well
- Earthing Busbar
- Copper Tapes
- Cable
- Solar System
- Cathodic Protection System
- بررسی کلی محصولات
- محصولات
- کتابخانه
- درباره ما
- تماس با ما